EVEN AFTER DECADES OF RESEARCH ON THE PATHOGENESIS OF ALZHEIMER’S DISEASE (AD) IT’S CAUSE ELUDES THE MEDICAL COMMUNITY AND RESEARCHERS HAVE NOT BEEN ABLE TO FORM LINKS BETWEEN ATROPHIC CHANGES OCCURRING IN THE BRAIN.
According to T3D Therapeutics Inc., a privately-held, Research Triangle Park, NC region-based company, these atrophic changes consist of “A multitude of cellular, biochemical and molecular abnormalities that have been observed including loss of cells, beta amyloid deposits, neurofibrillary tangles, activation of cell death genes, deficiencies in energy metabolism, mitochondrial dysfunction, DNA damage and increased oxidative stress.”
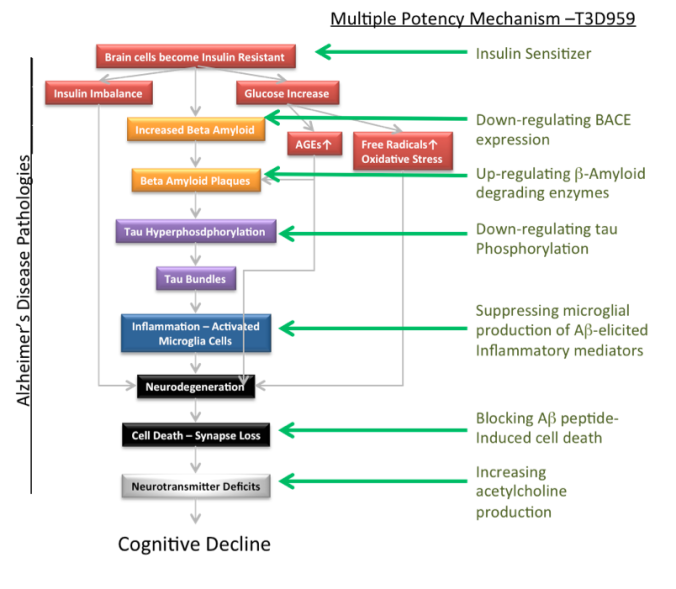
However, since 2005, a large body of evidence is pointing to insulin resistance and insulin deficiency as the ‘trigger’ for the above abnormalities.
T3D Therapeutics is on it.
Incorporated in 2013, T3D Therapeutics’ mission is to “develop and commercialize an optimal therapeutic for the treatment of Alzheimer’s disease and mild cognitive impairments.”
It has developed and has a worldwide license for “T3D-959 and structurally related molecules” to treat AD on the basis that it is Type 3 Diabetes.
Not only has T3D Therapeutic developed T3D-959, it’s been through pre-clinical trials, a Phase 1 clinical trial and on June 22, 2015 it received approval from the Division of Neurology Products of the U.S Food and Drug Administration to begin a Phase 2a clinical study clinical study of TsD-959 in 36 patients.
T3D Therapeutics Inc. T3D-959
T3D959 is a promising new, potentially disease-modifing therapeutic for AD. It is shown multi-faceted effectiveness on memory, motor function, inflammation, neuronal cell death, beta amyloid production and tau alteration in its pre-clinical trial involving drug-treated Streptozotocin-treated Alzheimer’s rats.
Essentially, according to the T3D Therapeutics website, “The earliest abnormality of cognitive impairments are those involving sugar (glucose) utilization.
The brain is nearly dependent on glucose as an energy source and a reduction of brain glucose metabolism is a hallmark of the disease.
Clinical symptoms of AD never occur without decreases in glucose metabolism.
Insulin the the key hormone responsible for efficient utilization of glucose by brain cells. Without the ability to use insulin to access glucose as an energy source neurons die. The result is cognitive and functional decline.”
T3D-959 is a small molecule dual nuclear receptor agonist being tested which has insulin-sensitizing activity. The drug helps correct insulin resistance in the brain and has excellent safety and pharmaceutical attributes.
It will be taken by the 36 patients in capsule form once per day for two weeks.
This is over the top, but patients will be monitored for cerebral glucose metabolism by FDG (fluorodeoxyglucose) – PET and for neural connectivity by BOLD (Blood oxygenation level dependent imaging) fMRI (functional magnetic resonance imaging).
BOLD monitors the flow of blood and fMRI observes different areas of brain activity.

FDG-PET Imaging – Measuring Cerebral Glucose Metabolism (CMRgl)
[warm-colored areas indicate higher glucose metabolism]
The upshot, is that in Barrons, in an article by Andrew Bary, “The Fight Against Alzheimers: Is hope Near?” he writes “some think treatment or treatments for Alzheimer’s disease could generate $ 10 billion to $ 20 billion in annual sales.”
According to bridgingstars.org: “Currently, there are 5. 3 million Americans living with AD, due to increasing life expectancy and lifestyle changes this number is projected to triple by the year 2050.”
In a News Release published on December 7, 2015 T3D Therapeutics announced it was presenting a company update at the 1st Annual Neuroscience BioPartnering and Investment Forum conference in New York City. The presentation was held at the New York Academy of Sciences on February 23, 2016.
On January 13, 2016 T3D Therapeutics presented a company update at the 8th Annual Biotech Showcase conference in San Francisco.
Too bad they are a private company.

http://www.t3dtherapeutics.com
(Sources: National Institute on Aging – http://www.t3dtherapeutics.com – http://bridgingstars.org/type-3-diabetes-the-epidemic/